Expert Medical Writing & Regulatory Documents
for Clinical, Trade Publication & Physician Audiences
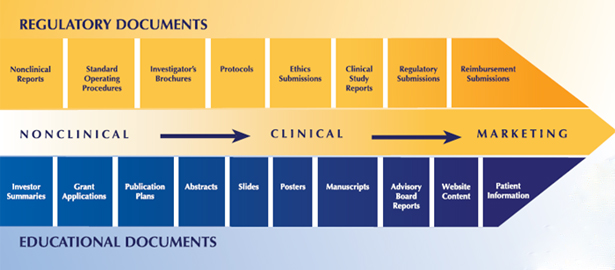
Science Author, Inc. provides expert medical writing, research, slide deck preparation and regulatory writing in a broad spectrum of therapeutic areas, including oncology, neurology, immunology, respiratory and ophthalmology and more. The scope of our medical writing services includes FDA briefing books and regulatory affairs documentation, clinical journal publications, continuing medical education, and medical meeting presentations. We also design, prepare, facilitate, survey and report on medical meetings of all types and sizes, from regional advisory groups to large international trade shows. If you need assistance in any of these or related areas, contact us for a free consultation and proposal for your project.